Abstract
Background: Therapy-related myeloid neoplasm (t-MN) is considered a direct consequence of cytotoxic therapy induced DNA damage in hematopoietic stem cells. Despite increasing recognition that altered stroma can also drive leukemogenesis, the functional biology of the t-MN bone marrow (BM) microenvironment remains unknown. We have previously shown that t-MN BM mesenchymal stromal cells (BMSC) are highly senescent and exhibit characteristic flattened shape, high p21 and β-Galactosidase expression, delayed DNA repair and secrete senescence associated cytokines and chemokines (Kutyna et al ASH 2021).
Aim: To assess reversibility of senescent t-MN BM stroma.
Methods: We performed functional and mechanistic evaluation of aberrant t-MN BMSC with and without senescent inhibitors. Results: Despite senescent state, t-MN BMSC were metabolically highly active with a switch towards glycolysis. Notably, the majority of ATP in t-MN BMSC was generated by glycolysis (69% vs. 36%; P <0.001), contrasting with healthy BMSC in which ATP was predominantly generated by mitochondrial oxidative phosphorylation (OXPHOS) (64% vs. 31%; P <0.001). The switch towards glycolysis, away from OXPHOS could be due to compromised mitochondrial function as indicated by high level of reactive oxygen species (ROS) in t-MN BMSC. We next assessed whether glycolytic ATP production was hardwired in t-MN stroma or could be switched to OXPHOS. As expected, glycolysis inhibitor, 2-Deoxy-D-glucose (2DG) significantly reduced glycolysis (P <0.001) in healthy and t-MN BMSC, but without significant reduction in total ATP, indicating a retained capacity to upregulate OXPHOS.
Despite being highly metabolically active and dependent on glycolysis, t-MN stromal cells display defective adipogenesis. To understand the mechanism of defective adipogenesis in t-MN, we first focused on known inhibitors of adipogenesis from cytokine bead array data. We found secretion of the adipogenesis inhibitory cytokines, IL-1β, IL-15, IL-6, IFNα and IFNγ were significantly higher by t-MN compared to healthy BMSC and noted a significant overlap with pro-inflammatory cytokines known to be senescence-associated-secretory phenotype (SASP) cytokines (P <0.001). To confirm this experimentally, treatment of healthy BMSC with IL-1β, IFNγ, IFNα or a cocktail of seven cytokines (IL-1β, IL-13, IL-15, IL-6, IFNα and IFNγ) profoundly inhibited adipogenesis (P <0.003), demonstrating a potential causative role of SASP in inhibiting adipogenesis. We further tested whether the bioenergetic phenotype observed in t-MN may also contribute to the defective adipogenesis. Blockade of either mitochondrial OXPHOS or glycolysis in healthy BMSC led to significantly reduced adipogenesis (P <0.001) consistent with the metabolic requirement of adipogenesis to store glucose-derived de novo fatty acids and mitochondria fatty acid β-oxidation. We then assessed whether switching glycolytic bioenergetic phenotype to OXPHOS would restore the adipogenesis in t-MN, however glycolysis inhibitor (2DG) did not restore defective adipogenesis in t-MN BMSC, suggesting that reversing aberrant metabolism less likely to restore functional capacity of t-MN BMSC.
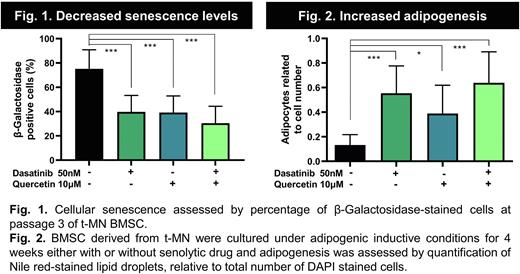
We hypothesise that defective functional capacity of t-MN BMSC is driven by high cellular senescence. Therefore, the next set of experiments assessed whether senescence drives the aberrant differentiation potential of t-MN BMSC by treating t-MN BMSC with senolytic agents and assessing their differentiation potential in in vitro culture. Senolytic agents, Dasatinib (75 ±16 vs. 40 ±14%; P <0.001), Quercetin (75 ±16 vs. 39 ±14%; P <0.001) alone or in combination (75 ±16 vs. 30 ±14%; P <0.001) significantly reduced senescence burden of t-MN BMSC (Fig. 1) and restored normal differentiation capacity of non-senescent t-MN BMSC, as reflected by six-fold increase in adipogenic potential (0.13 ±0.08 to 0.64 ±0.25; P <0.001) (Fig. 2). Moreover, senolytics also reduces aberrantly excessive osteogenic potential to near normal (277 ±40 vs. 108 ±45 Ca2+/µg DNA; P <0.001).
Conclusion: Collectively our results suggest that t-MN stroma is highly senescent yet metabolically very active. Importantly, senolytic therapies can effectively reduce senescence burden and restore differentiation potential of non-senescent t-MN BMSC, suggesting possible regenerative therapies for restoring defective BM microenvironment.
Disclosures
Hughes:BMS: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Hiwase:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Speakers Bureau; Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal